Breakthroughs
Targeting Pre-Eclampsia Helps To Close Gap in Health Equity
Pre-eclampsia is a leading cause of stillbirths and preterm births worldwide, occurring in 3% to 8 % of pregnancies. A disorder characterized by high blood pressure in pregnancy, it results from insufficient vasodilation in the placenta, restricting blood flow to the fetus.
Currently, a health care plan for patients with pre-eclampsia involves diet and movement changes, frequent monitoring, blood pressure management, and can mean early delivery of the baby. These standards of care address symptoms of the condition, not the root cause, and further perpetuate inequities that stem from a lack of health care access.
A team of researchers, led by Michael Mitchell, Associate Professor in Bioengineering, and Kelsey Swingle, lead author and doctoral student in the Mitchell Lab, is hoping to close this longstanding gap in reproductive health care with targeted RNA, which could one day address the lack of therapeutics to stop or slow the progression of pre-eclampsia once it is diagnosed.
“Current health care for patients with pre-eclampsia is lacking,” says Swingle. “Many times, the only thing doctors have been able to do is plan for an early delivery, resulting in premature birth, which comes with associated challenges. The status quo only worsens the health of patients and their babies in instances where prenatal and premature care are limited.”

“In pre-eclampsia, babies that are born early can be affected with stunted or abnormal growth and physiological development. A treatment that resolves the issue at the source would allow for higher quality of life and health for patients and their babies over the long term. Addressing this research gap is one way I can be an advocate for women’s health equity.” – Kelsey Swingle
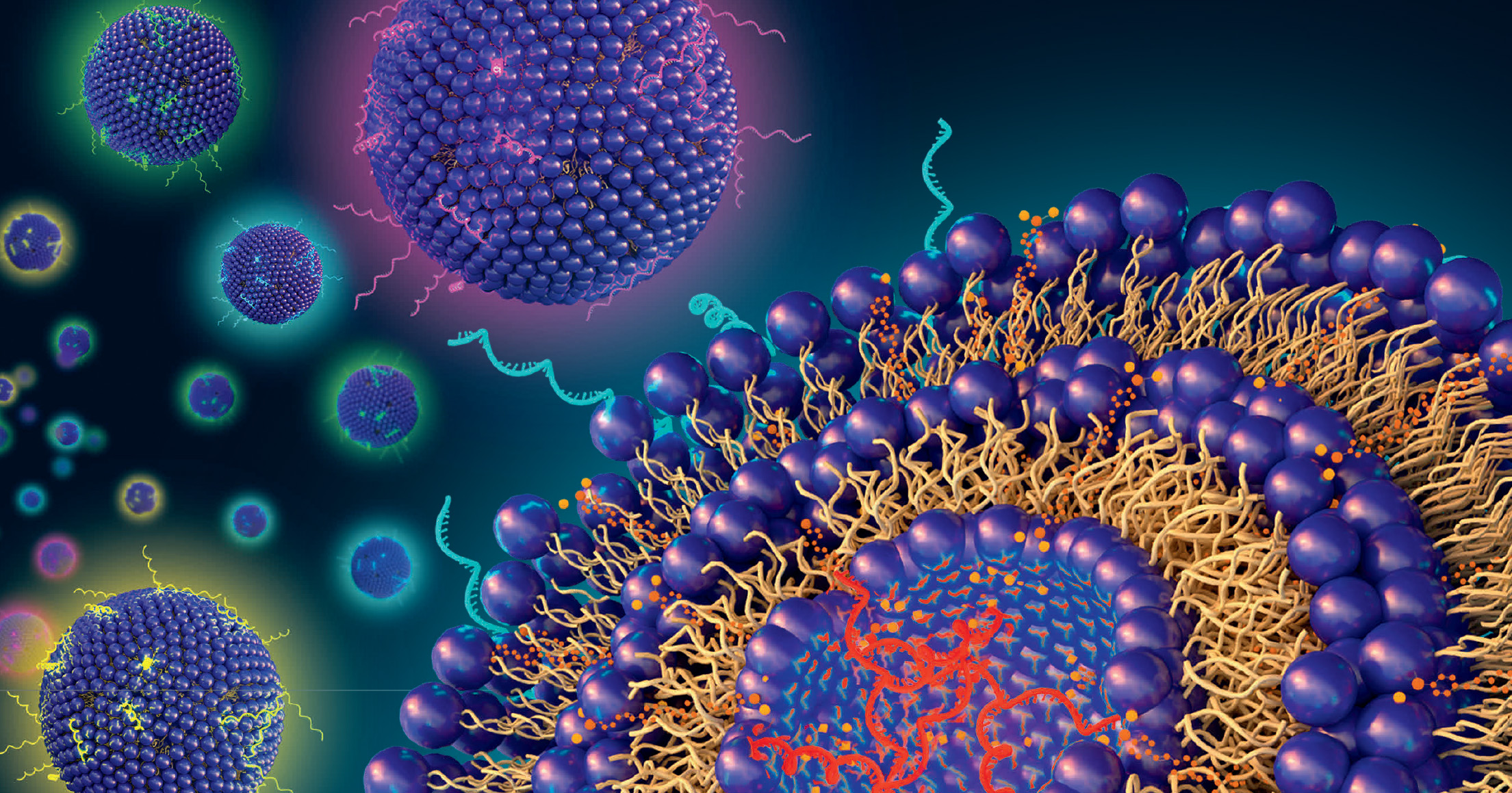
The development of the COVID vaccines demonstrated how lipid nanoparticles (LNPs) efficiently deliver mRNA to target cells. The success of LNPs is opening doors for a variety of RNA therapies aiming to treat the root causes of illness and disease. However, drug development and health care have consistently neglected a portion of the population in need of targeted care the most: pregnant people and their babies.
In one of the first studies of its kind, the Penn Engineering team developed an LNP with the ability to target and deliver mRNA to trophoblasts, endothelial cells and immune cells in the placentas of pregnant mice.
Once these cells receive the mRNA, they create vascular endothelial growth factor (VEGF), a protein that helps expand the blood vessels in the placenta to reduce blood pressure during pregnancy and restore adequate circulation to the fetus. The researchers’ successful trials in mice may offer a promising future for pre-eclampsia treatments in humans.
This is the first time LNP-assisted delivery of mRNA to the placenta has been shown.
“With no previous research to start from, our first challenge was to figure out which LNPs would actually travel to and target the placenta,” says Swingle. “We started by creating a library of LNPs using our knowledge from the work we did on LNP delivery to the liver. It turns out the liver and the placenta are actually very similar. They both receive high blood flow and contain intricate trees of blood vessels.”
By pairing a subject’s natural blood flow to the womb with a highly specific ionizable lipid in the LNP, the research team was able to target and deliver VEGF to placental cells in pregnant mice via a simple tail vein injection.
“The COVID vaccines were administered as intramuscular injections, a shot in the arm,” says Swingle. “This treatment would be administered intravenously. That means a patient would be able to be treated via a simple, noninvasive, and pain-free IV drip.”
Pregnant people were neglected in the clinical trials for the COVID vaccines, leaving many uncertain about how to safeguard their health and that of their babies. This oversight is not new. The majority of drugs on the market have not been tested in pregnancy, and disorders during pregnancy are frequently untreatable before birth.
The treatment presented in this groundbreaking study contributes to addressing health inequities for women, a major motivator for Swingle.
“I was inspired to research targeted therapies for maternal and fetal health in the spring of 2021 when people were making decisions about getting the COVID vaccine and patients had questions about safety that we could not answer,” she says. “In pre-eclampsia, babies that are born early can be affected with stunted or abnormal growth and physiological development. A treatment that resolves the issue at the source would allow for higher quality of life and health for patients and their babies over the long term. Addressing this research gap is one way I can be an advocate for women’s health equity.”
The new approach to LNP development for RNA therapies is also opening new doors for hard-to-treat diseases. This study is just one example of where this work is headed.
“Researchers have been doing a lot of work on the mechanisms and movements of drugs into cells, but there is not a lot out there on how therapies can be targeted to treat the root cause of diseases and conditions, particularly during pregnancy,” says Mitchell. “We now have a new LNP platform and can plug and play different RNA therapeutics to begin to develop successful therapies for many pregnancy conditions. This work is leaping off that platform, and we are excited to take the next steps.”
Story by Melissa Pappas, originally published on Penn Engineering Today / Photo of Kelsey Swingle by Kevin Monko
Inside Engineering
Fat Droplets Reveal DNA Danger

“Intuitively, people think of fat as soft. And on a cellular level it is. But at the small size of a droplet, it stops being soft. It can deform. It can damage. It can rupture.”
Dennis Discher
The fat-filled lipid droplets (pictured in yellow) found inside different cell types are tiny spheres many times smaller than actual fat cells. The lab of Dennis E. Discher, Robert D. Bent Professor in the Departments of Chemical and Biomolecular Engineering, Bioengineering, and Mechanical Engineering and Applied Mechanics, is the first to discover these droplets’ surprising capability to indent and puncture the cell nucleus, the organelle that contains and regulates a cell’s DNA. This groundbreaking work on the physics of these droplets reveals them to be a potential cause of the elevated DNA damage that is characteristic of many diseases, including cancer.

Using Kirigami in Breast Reconstruction
For breast cancer patients whose treatment includes mastectomy, complications caused by implant malposition can arise following reconstructive surgeries. In order to keep implants in place more effectively, the lab of Shu Yang, Joseph Bordogna Professor and Chair of Materials Science and Engineering, has used a papercutting technique known as kirigami to cover implants, shown here in a reconstruction model with a seamless architecture pattern made of white paper. Together with computational modeling, kirigami can provide surgeons with an easier-to-use, stretchable material that can better wrap around implants, thereby reducing the risk of malpositioning while also reducing the demand of the wrapping material that is usually derived from human tissue.

Understanding the Movement of Cholera
Though advances in sanitation have had a major impact in reducing infections from waterborne pathogens such as cholera, the illnesses remain a challenge, especially for populations without access to reliable sanitation infrastructure. Work led by Paulo Arratia, Professor in Mechanical Engineering and Applied Mechanics, uses fluid mechanics to understand and predict the movement of microscopic bacteria through flowing water. The group’s computational simulations and experiments with Vibrio cholerae create a path for technologies that both contain and disburse masses of bacteria before they can pose a threat to humans, laying the foundation for water security interventions.
Departmental Highlights
Bioengineering
New Single Cell Analysis Tool
The lab of Arjun Raj, Professor, has developed ClonoCluster, an open-source tool that combines unique patterns of gene activation with clonal information, producing hybrid cluster data that can quickly identify new cellular traits that can be used to better understand resistance to some cancer therapies.
Chemical and Biomolecular Engineering
Glassy Discovery
Work from Professors John Crocker and Robert Riggleman has shown that metadynamics, a computational approach to exploring energy landscapes, can be used to find rare low-energy canyons in glassy materials. This may have a wide range of useful applications, potentially reducing the computational cost of training future AIs.
Computer and Information Science
Securing the Census
Michael Kearns, National Center Professor of Management & Technology, and Aaron Roth, Henry Salvatori Professor of Computer & Cognitive Science, have demonstrated that U.S. Census statistics can be reverse-engineered to reveal protected information about individual respondents, affirming the need for enhanced “differential privacy” measures to protect personal data.
Electrical and Systems Engineering
Network Theory in Public Health
Shirin Saeedi Bidokhti, Assistant Professor, and Saswati Sarkar, Professor, have applied techniques from network and information theory to pandemic control and prevention, demonstrating the potential to deliver life-saving benefits while accommodating unequal access to medical resources, reluctance, budgetary constraints, testing errors and local practices.
Materials Science and Engineering
New Quantum Chip
The lab of Liang Feng, Professor, has created a chip containing a hyperdimensional microlaser that outstrips the security and robustness of existing quantum communications hardware. Their technology communicates in “qudits,” doubling the quantum information space of any previous on-chip laser and makes for robust quantum communication technology better suited for real-world applications.
Mechanical Engineering and Applied Mechanics
Squeezing Molecules To Reduce Waste
Robert Carpick, John Henry Towne Professor, has collaborated to produce a novel mechanochemistry method to manufacture existing and even new industrial chemicals that uses organic chemistry and nanotechnology to push molecules together, creating those chemicals without the need for costly solvents that pollute the environment.
