
In Practice
Building Tiny Organs
More than 34 million Americans suffer from pulmonary diseases like asthma, emphysema and chronic bronchitis. While medical treatments can keep these ailments in check, there are currently no cures. Part of the reason, notes Dan Huh, is that it’s incredibly hard to study how these diseases actually work. While researchers can grow cells taken from human lungs in a dish, they cannot expect them to act like they would in the body. In order to mimic the real deal, it’s necessary to recreate the complex, 3D environment of the lung — right down to its tiny air sacs and blood vessels — and to gently stretch and release the tissue to simulate breathing.
Huh, Associate Professor in Bioengineering, is the cofounder of Vivodyne, a Penn Engineering biotech spinoff that is creating tissues like these in the lab. Vivodyne uses a bioengineering technology that Huh has been developing for more than a decade. While a postdoctoral fellow at Harvard’s Wyss Institute, he played a central role in creating a novel device called an “organ on a chip,” which, as the name implies, assembles multiple cell types on a tiny piece of engineered plastic to create an approximation of an organ.
“While those chips represented a major innovation,” says Huh, “they still weren’t truly lifelike. They lacked many of the essential features of their counterparts in the human body, such as the network of blood vessels running between different kinds of tissue, which are essential for transporting oxygen, nutrients, waste products and various biochemical signals.”
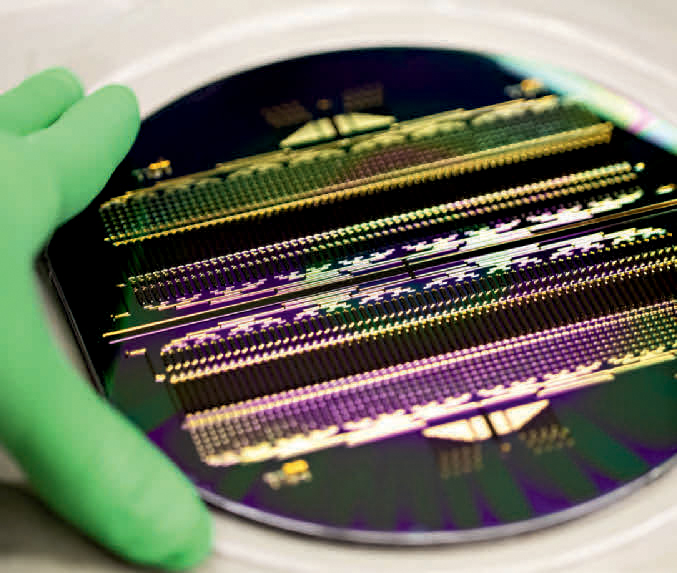
Vivodyne’s latest organ-chip technology puts hundreds of individually addressable culture chambers on a single 6-inch, disc-shaped device, meaning users can run 256 independent experiments simultaneously.
At Vivodyne, Huh is taking a biologically inspired approach to making realistic tissue, making the cells do the work themselves. The team places progenitor cells (an immature cell type that can become any kind of specialized cells present in the organ from which they are derived) into a gel-like media designed to provide the environment reminiscent of that seen in naturally developing tissues. Using this bioengineered, body-like environment, the team can jump-start the progenitor cells, steer their development into different types of specialized cells and gradually let them self-assemble into tiny organ-like structures.
“Optimizing this process is a lot of work,” says Huh. “But the end result is that we can now reverse-engineer these beautifully complex human tissues in plastic devices the size of a memory stick that we can use for a wide variety of scientific experiments.”
Using their unique method, Vivodyne can grow structures that replicate either healthy organs or those with a specific disease. The team has already created a variety of models that mimic the functional elements of different organs, such as human lungs, liver, brain, gut and pancreas, and have even used their process to create an artificially grown human eye with a bioengineered cornea, artificial tear ducts and a mechanical sliding eyelid. Together, these advances provide an innovative and robust way to study diseases as disparate as cancer, diabetes and dry eye syndrome.
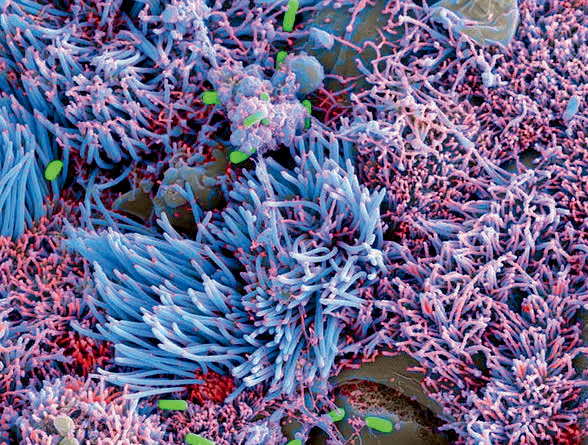
Vivodyne’s model of the human lung airways allows scientists the opportunity to study aspects of complex diseases, like chronic bacterial infection by Pseudomonas bacteria in the lungs of patients with cystic fibrosis. (Image courtesy of Vivodyne)
Now, Vivodyne is working to take this technology to the next level. “This is still largely confined to academic research,” says Huh. “The engineering complexity of organ-chip prototypes developed in academic labs poses major challenges to conducting a large number of simultaneous experiments in a reproducible manner. This problem makes it difficult to generate sufficient amounts of data often required for real-world applications. Resolving this fundamental issue was the main reason we started Vivodyne.”
To address the throughput issues, Huh and Vivodyne are working to put hundreds of identical mini organs on a single chip, a development that could let scientists quickly study a wide range of diseases and test new drugs to treat them. “Using our new technology, we can create up to 256 individually addressable culture chambers on a single 6-inch, disc-shaped device, meaning users can run 256 independent experiments simultaneously,” says Huh. “We can also stack five or more of these devices together, which adds up to more than 1,000 concurrent experiments. That’s orders of magnitude higher than the current state of the art.”
Vivodyne also offers end-to-end automation with a machine containing a computer-controlled, industrial robotic arm and microscopy to automate the entire process of tissue engineering and analysis in real time without human error. Using AI and machine learning-based approaches, the team is working to harness the power of the large amounts of high-quality biological data generated by these models.
Vivodyne’s approach has led to collaborations with several major pharmaceutical companies, including GlaxoSmithKline, AstraZeneca and Genentech, to conduct pilot studies of its technology. It’s a big vote of confidence for the young company, and Huh is quick to point out that his colleagues in the University have played a critical role in its success.
“Developing this technology is a great example of the opportunity for interdisciplinary collaboration at Penn,” he says. “Being part of Penn Engineering has played an indispensable role in the success of our work to date, and I look forward to continuing to enjoy and benefit from this rich environment.”
Text by David Levin / Photos by Leslie Barbaro
